
At high temperatures, molecules may disassociate into atoms, and atoms disassociate into electrons (with negative charges) and protons (with positive charges), forming a plasma. There exists one other phase of matter, plasma, which exists at very high temperatures. In this chapter, we generally refer to both gases and liquids simply as fluids, making a distinction between them only when they behave differently. When placed in an open container, gases, unlike liquids, will escape. This makes gases relatively easy to compress and allows them to flow (which makes them fluids). In contrast, atoms in gases are separated by large distances, and the forces between atoms in a gas are therefore very weak, except when the atoms collide with one another. Because the atoms are closely packed, liquids, like solids, resist compression an extremely large force is necessary to change the volume of a liquid. When a liquid is placed in a container with no lid, it remains in the container. That is, liquids flow (so they are a type of fluid), with the molecules held together by mutual attraction.
#Density equation free#
This occurs because the atoms or molecules in a liquid are free to slide about and change neighbors. Liquids deform easily when stressed and do not spring back to their original shape once a force is removed. A gas must be held in a closed container to prevent it from expanding freely and escaping. (c) Atoms in a gas move about freely and are separated by large distances. Forces between the atoms strongly resist attempts to compress the atoms. (b) Atoms in a liquid are also in close contact but can slide over one another. Oil floats on vinegar because its density is lower.Figure 14.2 (a) Atoms in a solid are always in close contact with neighboring atoms, held in place by forces represented here by springs.Over time, the helium escapes the balloon and is replaced by air, causing it to sink. Helium balloons rise because helium is less dense than the surrounding air.All that matters is the relative densities of the substances.
Scientifically, what counts isn’t the fact that one is wood and the other stone. Many widely used hardwoods, such as ebony, mahogany and lignum vitae, are dense enough to sink in water, and a few rocks, such as pumice, are light enough to float. This obvious example illustrates the power of science in real life. Rocks, generally being denser than water, usually sink.

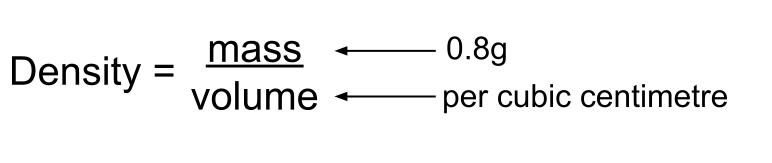
This formula can be used to determine the density of any substance.

That is, density (p) is equal to total mass (M) divided by total volume (v). Density is calculated according to the simple formula:


 0 kommentar(er)
0 kommentar(er)
